Portrait of the Month
Prof. Dr. Ivan Dikic, MD, PhD
Professor and Chairman of Institute of Biochemistry 2
Founding Director of the Buchmann Institute for Molecular Life Sciences
Group Leader
Goethe University Medical School Frankfurt am Main
Email: dikic@biochem2.uni-frankfurt.de
Homepage 1: http://www.biochem2.com/molsig/projects.html
Homepage 2:
http://www.ncbi.nlm.nih.gov/pubmed/?term=Dikic+I[Author]
Homepage 3:
http://www.biochem2.com/molsig/Ivan_Dikic.html
Video Titel Lorem Ipsum
News:
Ivan Dikic is a Professor and Chairman of Institute of Biochemistry 2 at the Goethe University Medical School and Founding Director of the Buchmann Institute for Molecular Life Sciences in Frankfurt. Before joining Goethe University he was a Group Leader at the Ludwig Institute for Cancer Research, Uppsala, Sweden. He was trained as a medical doctor in Zagreb, Croatia and obtained his PhD in molecular biology from the University of Zagreb and New York University Medical Center.
His research focuses on the role of ubiquitin (Ub), a small protein that is covalently attached to thousands of cellular proteins. His pioneering work explained how Ub acts as a multivalent cellular signal that is recognized by an expanding number of Ub-binding proteins, which in turn translate Ub code into appropriate cellular phenotypes. His group demonstrated the importance of Ubiquitin in the regulation of DNA repair, inflammation, cancer, infection, receptor endocytosis, and proteasomal degradation. They revealed mechanisms by which linear ubiquitination can regulate the NF-kB pathway making a decision between cell survival and death pathways. His current interests focus on the role of ubiquitin in selective autophagy, which is essential for the clearance of protein aggregates, pathogens, and damaged mitochondria from the cell.
He has been awarded the AACR Award for Outstanding Achievement in Cancer Research in 2006, the Hans Krebs Prize 2010, the Leibniz Award 2013 and Ernst Jung Award in Medicine 2013. He is a member of the German Academy for Sciences Leopoldina and EMBO.
Current Research: Lorem ipsum dolores est
Brückentext: Lorem Epsom is a Professor and Chairman of Institute of Biochemistry 2 at the Goethe University Medical School and Founding Director of the Buchmann Institute fo
Autophagy was first described as cellular response to starvation and describes the general process of delivery of cargo to lysosomes for degradation.Macroautophagy (henceforth „autophagy“), is the most prevalent form and involves the formation of double-membrane vesicles (autophagosomes) that sequester portions of the cytoplasm and eventually fuse with the lysosome, where their cargo is degraded (Klionsky, 2005).
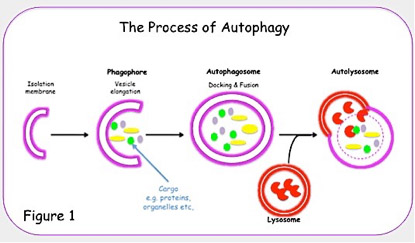
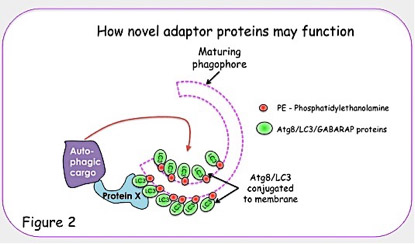
Our group is currently interested in the discovery of novel autophagy receptors important for selectivity of cargo delivery, as well as delivery and fusion of mature autophagosomes to the lysosomes.
Using the ubiquitin-like autophagy proteins, Atg8 (GABARAP, GATE-16 and LC3 in mammals), which can be conjugated to phosphatidylethanolamine (PE) in a process similar to ubiquitin conjugation, we carried out an extensive yeast-2-hybrid screen to identify novel receptors. Since Atg8 proteins are present on both the inner and outer leaflets of the isolation membranes, they are in a unique position to act as a platform for the recruitment of adaptor proteins that are important for the process of autophagy.
Current Research: Lorem ipsum dolores est
Brückentext: Lorem Epsom is a Professor and Chairman of Institute of Biochemistry 2 at the Goethe University Medical School and Founding Director of the Buchmann Institute fo
Current Research: Lorem ipsum dolores est
Brückentext: Lorem Epsom is a Professor and Chairman of Institute of Biochemistry 2 at the Goethe University Medical School and Founding Director of the Buchmann Institute fo
Previous work in our lab has identified NBR1 (Neighbour of BRCA1) as a novel autophagy receptor that can bind both Atg8-like proteins as well as ubiquitinated substrates. NBR1 has a similar domain structure to another known autophagy receptor, p62/SQSTM1; i.e. both contain a N-terminal PB1, a central Zinc finger and C-terminal UBA domain. We have shown that NBR1 can bind Atg8-like proteins, both in vitro and in vivo, which is dependent on a C-terminal LC3 interacting region (LIR) and NBR1 accumulates in an autophagy and LIR dependent manner (Kirkin et al. Mol. Cell. 2009).
Elimination of damaged mitochondria, as well as clearance of mitochondria during differentiation is regulated by a specialized type of autophagy, i.e. mitophagy (Shweers et al. 2007; Kundu et al, 2008; Sandoval et al, 2008; Zhang et al, 2009). Previously, Nix/BNIP3L protein has been reported to be important for the process of mitochondrial clearance and terminal differentiation of reticulocytes (Zhang & Ney, 2009).
In addition to NBR1, we identified Nix/BNIP3L as an interaction partner for autophagy specific UBL proteins LC3/GABARAP and implicated in mitophagy (Novak et al. 2009). We show that Nix binds to LC3 and GABARAP through two potential LIRs. One of them is particularly important since mutation in W35 was able to abolish Nix:LC3/GABARAP binding in vitro and mitochondrial clearance in vivo.
Dysfunction of autophagy contributes or can be a cause of many diseases and failure of autophagy can promote cancer, neurodegenerative disorders, liver disease, premature aging, inflammatory diseases, such as Crohn´s, and compromises host defense against pathogens. The identification of novel autophagy receptors, such as NBR1 and Nix, will allow us to gain a deeper understanding of the overall process of autophagy and in particular, the mechanisms governing selective autophagy and how it may be deregulated in neurodegenerative, inflammatory diseases as well as cancer.
Klicke den Bearbeitungs-Button um diesen Text zu verändern. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Klicke den Bearbeitungs-Button um diesen Text zu verändern. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.